Researchers at the University of Sheffield are embarking on a groundbreaking journey with the approval of the first human clinical trial for a regenerative cell therapy designed to treat sensorineural hearing loss. This innovative therapy, developed by Rinri Therapeutics, a spin-out company from the university, focuses on repairing damaged auditory neurons—one of the key factors behind this prevalent and often debilitating condition. Sensorineural hearing loss occurs when the delicate hair cells within the cochlea or the auditory nerve sustain damage, impairing the ability to hear. Currently, treatment options like cochlear implants are used to manage symptoms, but their effectiveness depends heavily on the remaining health of the auditory nerve, and no therapies exist that regenerate the damaged neurons themselves.
Rinri Therapeutics has developed a product called Rincell-1, an allogeneic regenerative cell therapy intended to restore the damaged auditory nerves and potentially improve hearing function. The upcoming clinical trial, approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA), will be a Phase I/IIa study involving 20 patients who will receive cochlear implants. Half of the patients will receive a single dose of Rincell-1 in addition to their implants. The trial includes two patient groups: those with postsynaptic auditory neuropathy spectrum disorder (ANSD) and those with severe-to-profound age-related hearing loss (presbycusis). Conducted at three leading UK hearing research centres, the study will assess safety and seek to identify changes in neural health, using advanced cochlear implant monitoring systems alongside speech perception tests and patient feedback.
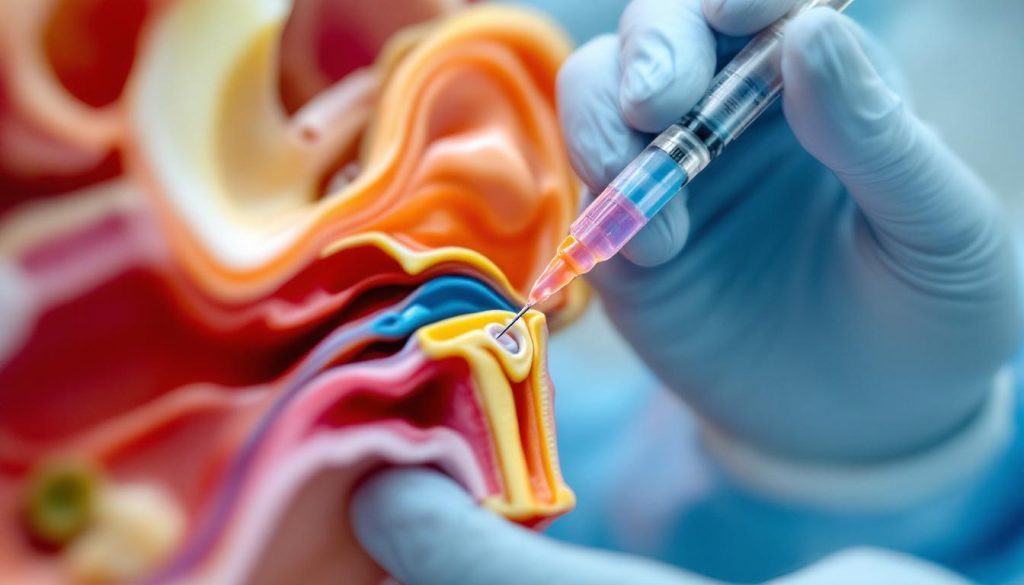
Delivering this therapy to the inner ear requires precision, which has been made possible through a novel minimally invasive surgical technique developed specifically for this trial. The method uses access via the inner ear’s round window, enabling controlled and safe administration of the regenerative cells during cochlear implant surgery. This approach not only ensures targeted delivery but also reflects recent advances validating safe access to the human cochlea’s central core, a critical barrier overcome in the development of regenerative therapies for hearing loss.
The scientific foundation of this therapy rests on years of research into sensory stem cell biology and regeneration at the University of Sheffield. The team has successfully established protocols to derive otic progenitor cells from human pluripotent stem cells, showing proof of concept in animal models where these cells have repaired damaged cochlea and restored auditory function. This advancement provides a critical preclinical basis for the promising cell therapy now moving into human trials.
According to Professor Marcelo Rivolta, founder and chief scientific officer at Rinri Therapeutics, gaining regulatory approval culminates years of dedicated scientific exploration and belief in regenerative medicine’s promise. The trial moves the field closer to a future where auditory function may be restored, not just managed. Simon Chandler, CEO of Rinri Therapeutics, highlighted the significant milestone this represents in addressing a major unmet medical need, thanking both the team and investors for their support. Chief medical officer and trial chief investigator Professor Doug Hartley emphasised the urgent need for therapies that can alter the course of hearing loss, describing the clinical trial as an exciting step forward in potentially regenerating auditory nerves and improving patients’ quality of life.
This trial represents a pioneering moment in hearing loss treatment, offering hope for the millions affected by sensorineural conditions. If successful, Rincell-1 could usher in a new era where regenerative therapies not only complement existing cochlear implants but fundamentally repair the underlying neural damage, transforming patient outcomes and advancing healthy ageing.
📌 Reference Map:
- Paragraph 1 – [1], [4], [6]
- Paragraph 2 – [1], [2], [3]
- Paragraph 3 – [1], [5], [7]
- Paragraph 4 – [6], [4]
- Paragraph 5 – [1], [3], [4]
Source: Noah Wire Services