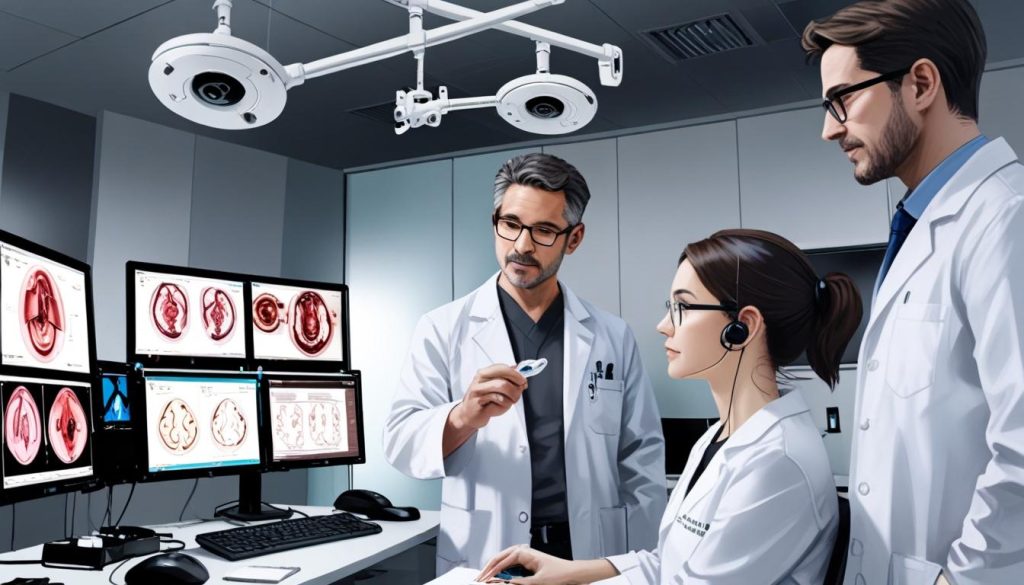
**Europe**: A recent study evaluates the TICI cochlear implant at hospitals in Belgium and Germany, focusing on participant safety, speech perception assessments, and patient satisfaction which could transform hearing restoration methodologies for those with severe hearing loss.
A recent study published by Nature.com has shed light on advanced methodologies in hearing restoration, particularly focusing on cochlear implantation techniques. This clinical trial assessed the feasibility of a cutting-edge cochlear implant, the TICI, at two prominent medical centres: the University Hospital Center of Liège in Belgium and the University Hospital of Munich in Germany.
The research emphasised stringent inclusion and exclusion criteria for participant enrolment. Eligible individuals were required to be at least eighteen years old, exhibit bilateral sensorineural hearing loss unresponsive to hearing aids, and demonstrate a specific level of auditory impairment as determined by recognised auditory tests. Participants were carefully screened to ensure optimal health conditions and realistic expectations regarding the treatment. Additionally, ethical standards were upheld, with each participant providing informed consent prior to the initiation of any procedures. The study received approval from ethics committees in both Belgium and Germany, and was registered under clinical trials.
A total of six individuals (four female) took part in the study, with enrolments commencing on 16 September 2020 and concluding on 16 December 2020. The demographic data revealed a mean age of 44.5 years among participants suffering from bilateral severe-to-profound hearing loss. The research aimed to monitor the occurrence of adverse events associated with the TICI implant and assess post-implantation speech perception in both quiet and noisy environments using standardised auditory tests.
Surgical procedures for the cochlear implants adhered to conventional practices, with the mean duration of surgery being 211 minutes. The study reported no significant complications during the postoperative recovery period. Initial activation of the TICI implant occurred four weeks post-surgery, allowing participants to transition from conventional audio processors to the innovative Cochlear Implant (CI). This transition aimed to facilitate comparative assessments of auditory performance between the TICI and traditional processors.
Speech perception testing was meticulously conducted, encompassing evaluations in quiet environments and amidst background noise. Participants underwent various testing protocols contingent upon the treatment site; these included the Freiburg Monosyllabic Test and the Monosyllabic Test of Fournier. These assessments provided insights into participants’ word recognition capabilities in varying auditory conditions.
In addition to objective measures, the study integrated patient-reported outcomes through the utilisation of four validated surveys. These aimed to capture participants’ self-reported quality of life and overall satisfaction with their auditory experience.
Despite the study’s limited sample size, the documentation serves as a critical step in the ongoing exploration of auditory healthcare technology. The findings hold promise for enhanced therapeutic avenues for individuals with hearing impairments, advancing the understanding of cochlear implants and their implications for auditory health and restoration.
The outlined measures within the study highlight the rigorous efforts undertaken to not only monitor the efficacy of the TICI implant but also to prioritise participant safety and satisfaction, reflecting a commitment to developing sophisticated solutions in hearing restoration.
Source: Noah Wire Services