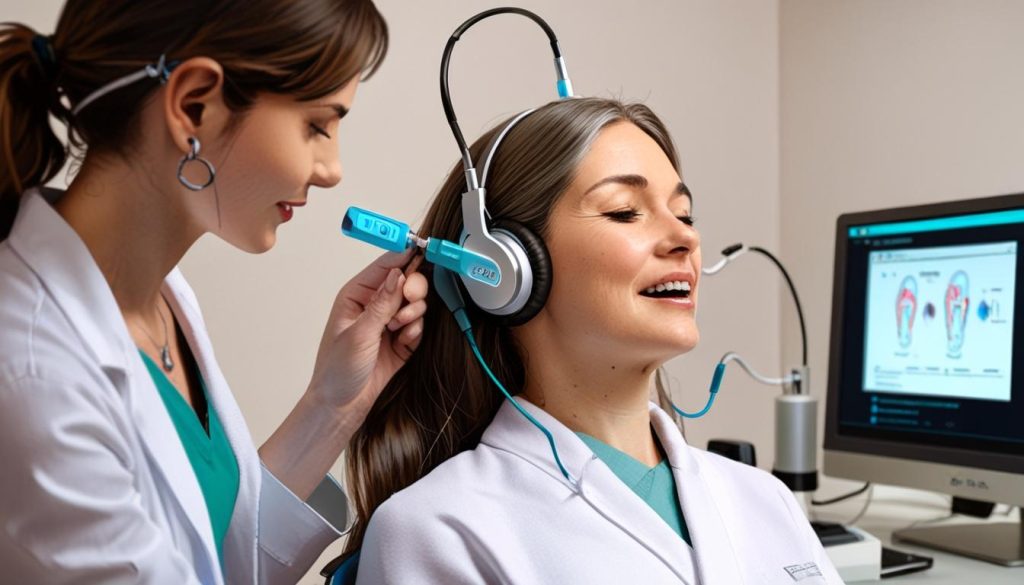
A recent study published in Nature Communications Medicine has shed new light on the effectiveness of the Lenire device, a pioneering treatment for tinnitus approved by the FDA. This research represents one of the largest real-world assessments conducted to date, involving 220 patients who were treated at the Alaska Hearing and Tinnitus Center between May 2023 and June 2024. According to the study, an impressive 91.5% of these patients reported a clinically significant reduction in their tinnitus symptoms after approximately 12 weeks of using Lenire.
Tinnitus, often described as a persistent ringing or buzzing in the ears, affects around 25 million adults in the United States alone. It is a condition that can greatly impact the quality of life, with many seeking effective treatment options. Lenire employs a unique method of bimodal neuromodulation, which simultaneously stimulates auditory pathways through sound while delivering electrical stimulation to the tongue. Treatment sessions typically last 30 minutes twice a day, under the oversight of an audiologist.
The retrospective analysis confirms the effectiveness of Lenire aligns with findings from previous large-scale clinical trials, particularly the TENT-A3 study that provided the basis for its FDA approval in March 2023. This earlier trial indicated that nearly 89% of participants would recommend Lenire as a treatment option. Additionally, the new study reported a mean improvement of 27.8 points on the Tinnitus Handicap Inventory (THI), further validating the efficacy of this innovative treatment approach.
Emily E. McMahan, Au.D., a leading audiologist involved in the study, commented, “The publication of these data in Nature Communications Medicine underscores the effectiveness of Lenire for patients suffering from tinnitus when they receive guidance from an experienced health professional.” This statement touches on the importance of professional oversight in maximising treatment benefits.
The safety profile of Lenire also appears favourable, with no serious adverse events related to the device reported in the study. Such findings further cement Lenire’s position as a promising solution for those battling chronic tinnitus. The positive outcomes echo earlier studies, which have revealed that bimodal treatment is superior to sound-only therapies, particularly for individuals with more severe symptoms.
Hubert Lim, Ph.D., a professor at the University of Minnesota and a pivotal figure in the research and development of Lenire, stated, “Obtaining positive real-world results that surpass those observed during our earlier controlled clinical trial represents a significant advancement in the field of tinnitus treatment.” His comments highlight the broader implications of the findings, suggesting a potential shift towards more effective, evidence-based therapies for tinnitus patients globally.
Lenire is currently available in specialised clinics across the United States and Europe, and it is also part of the treatment offerings provided by the U.S. Department of Veterans Affairs, addressing the needs of veterans who frequently experience tinnitus-related challenges.
The ongoing commitment of Neuromod Devices, the company behind Lenire, to evidence-based treatment and clinician support is pivotal as more data emerges from real-world settings. As the company continues to collect and publish findings from thousands of patients treated with Lenire, it aims to establish robust best practices that can enhance treatment outcomes for tinnitus sufferers.
As awareness grows around the condition and its treatments, the publication of these findings could signify a new era of tinnitus management, providing hope to millions seeking relief from this often-debilitating condition.
Reference Map
- Paragraphs 1, 2, 3, 4, 5, 6
- Paragraphs 1, 2, 4, 5, 6
- Paragraphs 1, 2, 4, 5, 6
- Paragraphs 1, 2, 4, 5, 6
- Paragraphs 1, 2, 4, 5, 6
- Paragraphs 1, 2, 4, 5, 6
- Paragraphs 1, 2, 4, 5, 6
Source: Noah Wire Services